One of chemistry introductions of iron
Opposite atomic mass 55.847. Iron has body of a variety of the different that be the same as element, the iron that be like α , β iron, γ iron, б iron. Iron is comparative and vivid metal, in the outside and the inside of metallic activity order the platoon is before hydrogen. When normal temperature, iron is not easy in as dry as a chip air with oxygen, sulfur, chloric wait for the echo since metalloid simple substance, when high temperature, acuteness and resonant. Iron burns in oxygen, generate Fe3O4, the echo since fervent iron and vapor also generates Fe3O4. In the inorganic acid of Tie Yirong Yu Xi and thick hydrochloric acid, generate diatomic molysite, give off hydric. Encounter below normal temperature thick vitriolic or
When iron thick nitric acid, the surface generates an oxide diaphragm, make iron " passivation " , reason can use ironwork full-dress thick vitriolic or thick nitric acid. Iron is element of one appraise at the current rate, common price condition is + 2 and + 3. Solution of iron and sulfur, bluestone, hydrochloric acid, rare vitriolic the lose when waiting for echo two electrons, become + 2 price. With Cl2, Br2, nitric acid and hot thick vitriolic echo, be become by oxidation Fe3+ . The Fe3O4 that iron and oxygen or vapor echo generate, can regard FeO · Fe2O3 as, the Fe that has 1/3 among them is + 2 price, additional 2/3 is + 3 price. Of iron + 3 price compound is relatively smooth and steady.
Article chapter comes from: Www.onsonhardware.com
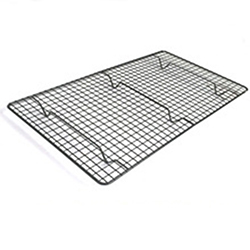 OSCAKT034370 46*26cm Footed Outdoor Rectangle Cooking Iron Metal Mesh Barbecue BBQ Grill Grid Ware Nets
OSCAKT034370 46*26cm Footed Outdoor Rectangle Cooking Iron Metal Mesh Barbecue BBQ Grill Grid Ware Nets OSCABS948 PE coated iron corner bathroom rack/corner bathroom stand/corner shelving for bathrooms
OSCABS948 PE coated iron corner bathroom rack/corner bathroom stand/corner shelving for bathrooms